Beta Sheet Entropy
Beta Sheet Entropy - Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within. Web conformational entropy of proteins: When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as.
Web conformational entropy of proteins: Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within. When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as.
Web conformational entropy of proteins: When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as. Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within.
Entropy 0.1.0 BETA file ModDB
Web conformational entropy of proteins: Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within. When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational.
Entropy Monthly Spezz Exchange
When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as. Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Unlike the α helix, the ß sheet is formed by.
ModelThinkers Entropy
Web conformational entropy of proteins: When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as. Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Unlike the α helix, the.
Entropy Network Medium
Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Web conformational entropy of proteins: Unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within. When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational.
entropy mediaVideo
When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as. Web conformational entropy of proteins: Unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within. Web beta sheets ß sheets the.
Information Transfer Economics II. Entropy and microfoundations
Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Web conformational entropy of proteins: When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as. Unlike the α helix, the.
What is entropy
Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within. When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and.
Entropy The Hidden Force That Complicates Life
Web conformational entropy of proteins: Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as. Unlike the α helix, the.
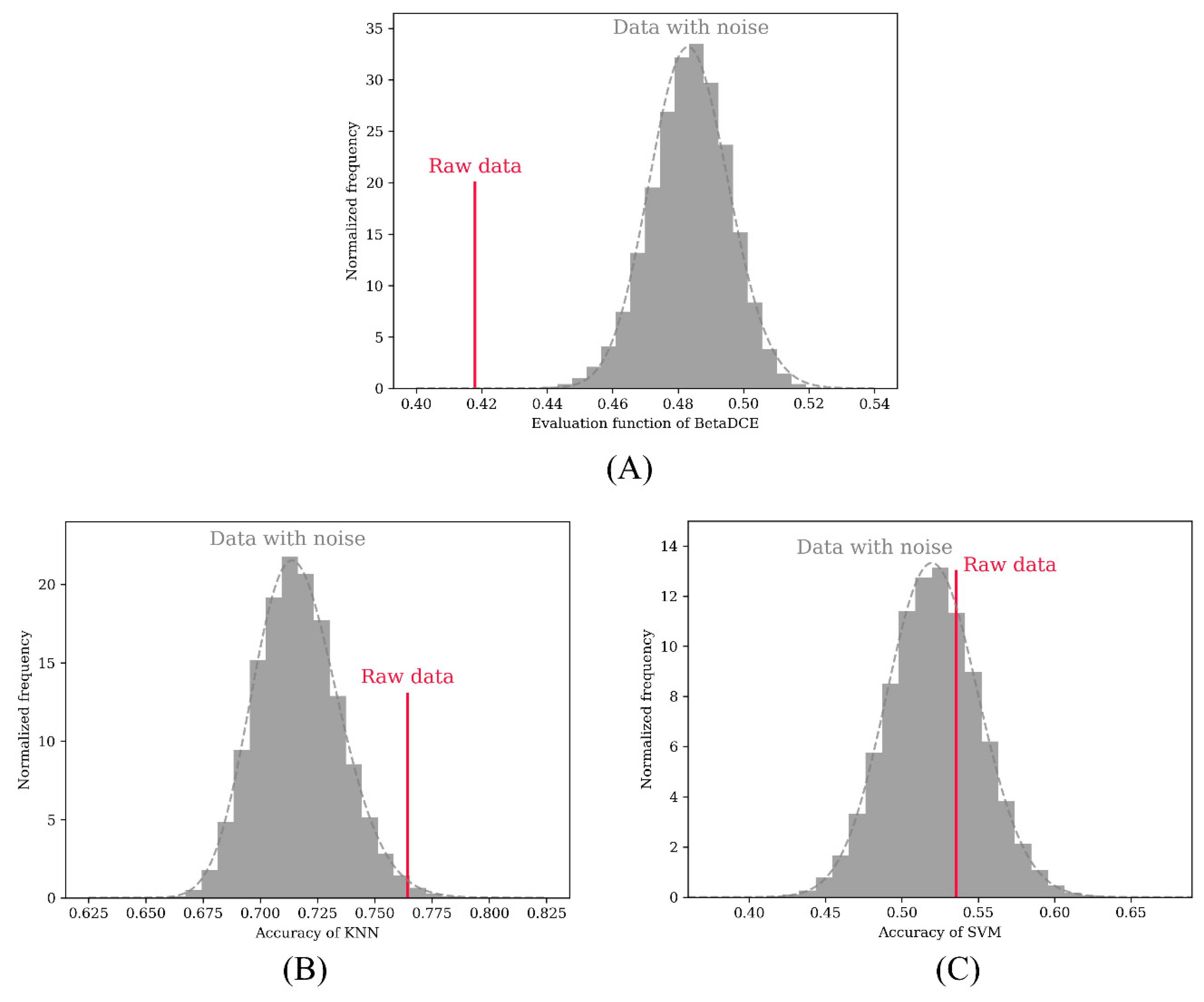
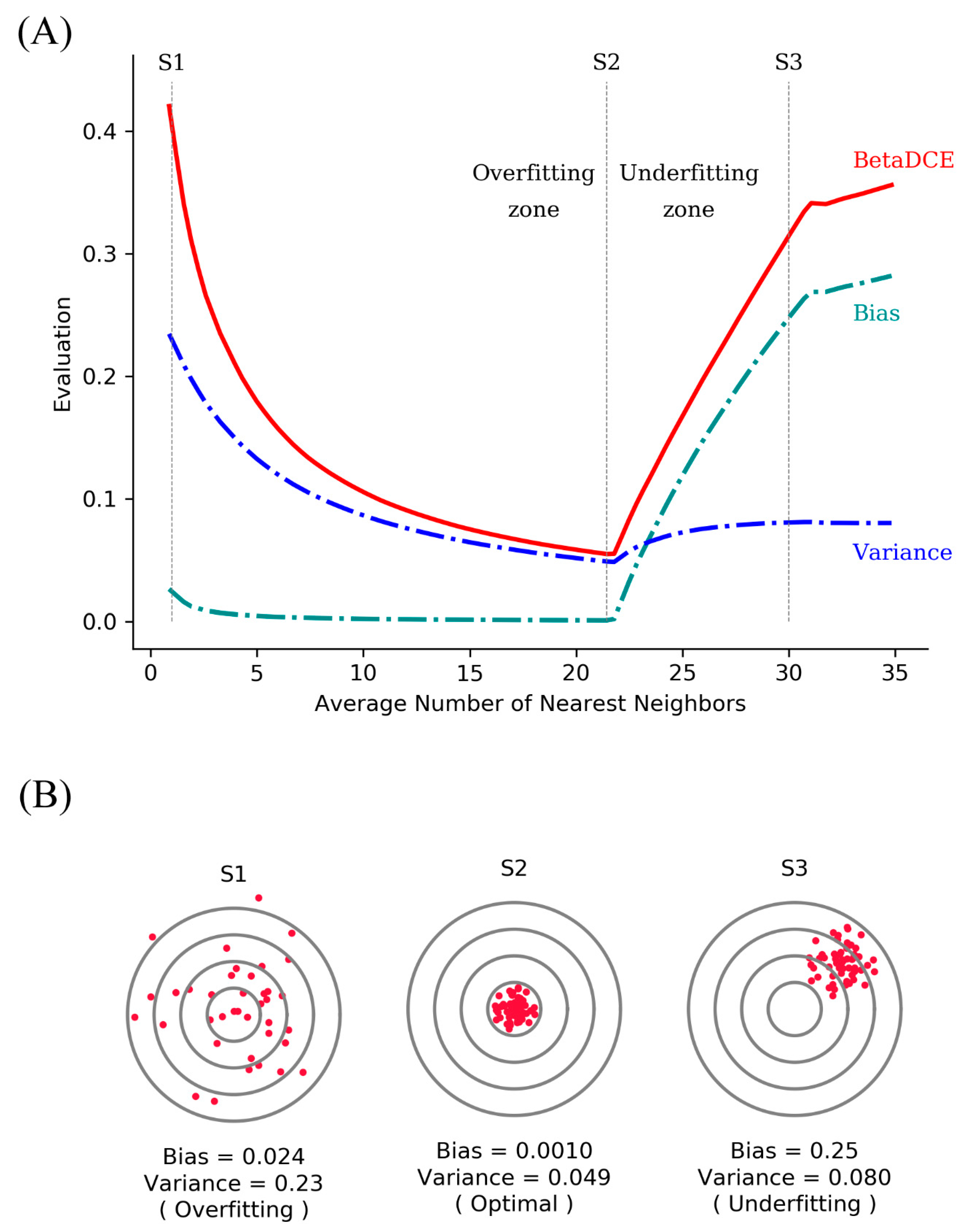
Entropy Free FullText Beta DistributionBased CrossEntropy for
When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as. Web conformational entropy of proteins: Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Unlike the α helix, the.
Entropy Free FullText Beta DistributionBased CrossEntropy for
Web beta sheets ß sheets the other type of secondary structure pauling and corey discovered is the ß sheet. Unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within. When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and.
Web Beta Sheets Ss Sheets The Other Type Of Secondary Structure Pauling And Corey Discovered Is The Ss Sheet.
Web conformational entropy of proteins: When a protein unfolds the entropy of the molecule increases dramatically due to a change in the conformational freedom of the phi and psi angles of the mainchain, as well as. Unlike the α helix, the ß sheet is formed by hydrogen bonds between protein strands, rather than within.