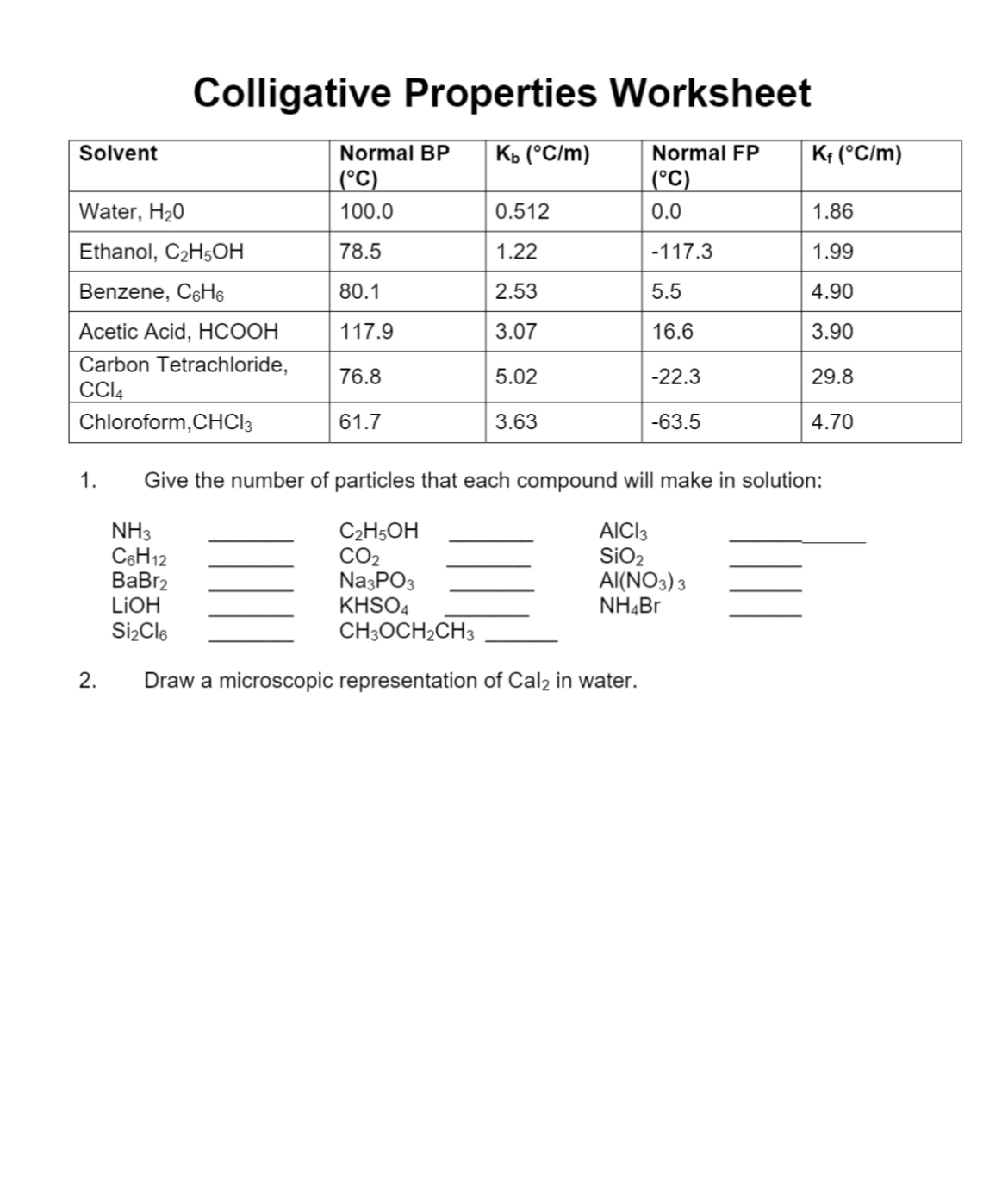
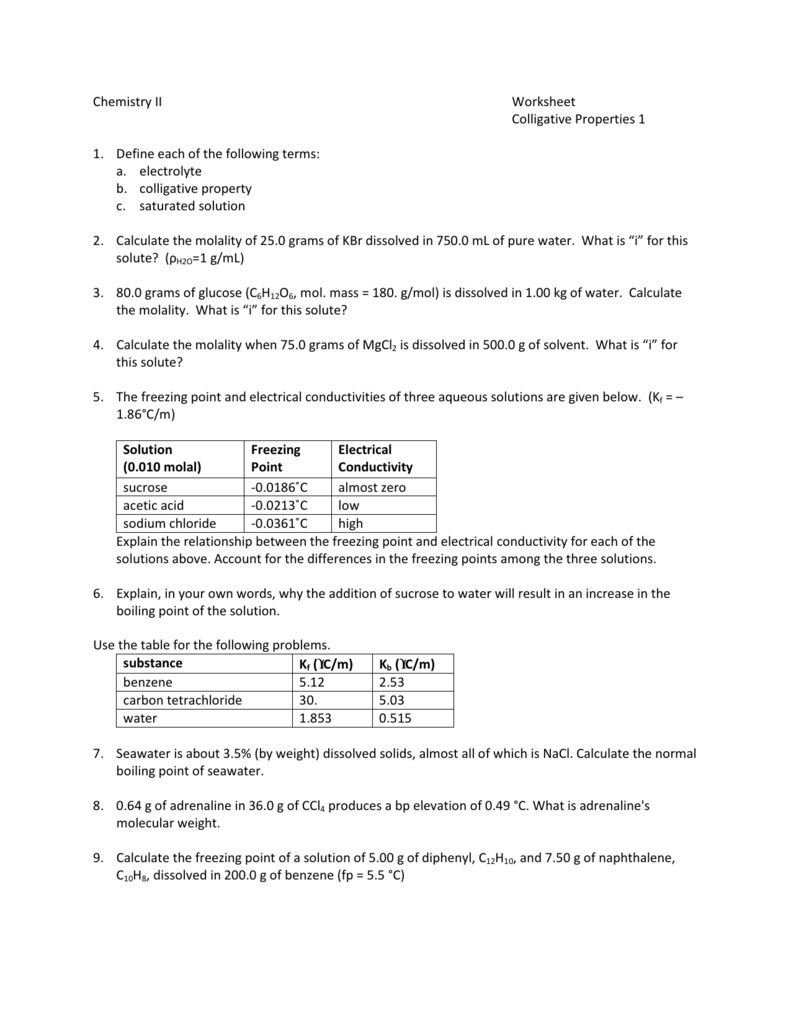
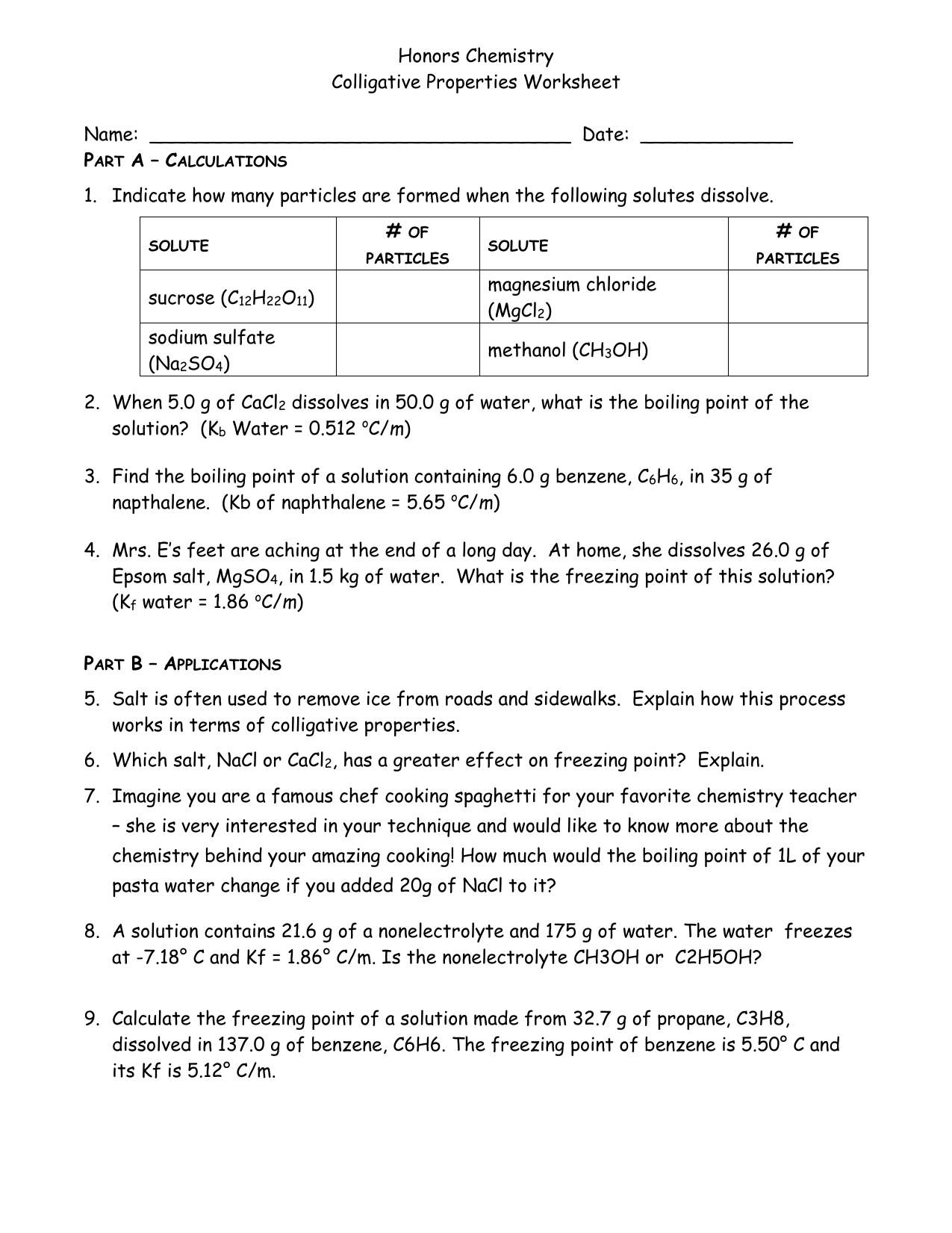
Colligative Properties Worksheet
Colligative Properties Worksheet - Give the molecular formula, the van’t hoff factor for the following ionic. Practice colligative properties and phase diagrams with this worksheet that includes problems and solutions. Find the solutions to three problems involving colligative properties of solutions, such as freezing point depression, boiling point. Colligative properties are properties that depend only upon the number of solute atoms, ions, or molecules in a solution and not on the nature of. The others you’ll most likely have to look up. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate.
Practice colligative properties and phase diagrams with this worksheet that includes problems and solutions. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. Colligative properties are properties that depend only upon the number of solute atoms, ions, or molecules in a solution and not on the nature of. The others you’ll most likely have to look up. Give the molecular formula, the van’t hoff factor for the following ionic. Find the solutions to three problems involving colligative properties of solutions, such as freezing point depression, boiling point.
Practice colligative properties and phase diagrams with this worksheet that includes problems and solutions. Find the solutions to three problems involving colligative properties of solutions, such as freezing point depression, boiling point. The others you’ll most likely have to look up. Colligative properties are properties that depend only upon the number of solute atoms, ions, or molecules in a solution and not on the nature of. Give the molecular formula, the van’t hoff factor for the following ionic. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate.
SOLUTION STEM Chemistry Colligative Properties of NonElectrolyte
The others you’ll most likely have to look up. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. Give the molecular formula, the van’t hoff factor for the following ionic. Colligative properties are properties that depend only upon the number of solute atoms, ions, or molecules in a solution and not on the nature.
Solved CHEMISTRY COLLIGATIVE PROPERTIES WORKSHEET Practice
Practice colligative properties and phase diagrams with this worksheet that includes problems and solutions. Give the molecular formula, the van’t hoff factor for the following ionic. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. Find the solutions to three problems involving colligative properties of solutions, such as freezing point depression, boiling point. The.
Solved Colligative Properties
Practice colligative properties and phase diagrams with this worksheet that includes problems and solutions. Colligative properties are properties that depend only upon the number of solute atoms, ions, or molecules in a solution and not on the nature of. Find the solutions to three problems involving colligative properties of solutions, such as freezing point depression, boiling point. The others you’ll.
Chemistry II Worksheet Colligative Properties 1 1. Define each of the
Find the solutions to three problems involving colligative properties of solutions, such as freezing point depression, boiling point. Colligative properties are properties that depend only upon the number of solute atoms, ions, or molecules in a solution and not on the nature of. The others you’ll most likely have to look up. We expect you to know the solubility of.
answerkey for chem Chapter 12 worksheet 4 (ws12) Colligative
Practice colligative properties and phase diagrams with this worksheet that includes problems and solutions. Give the molecular formula, the van’t hoff factor for the following ionic. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. The others you’ll most likely have to look up. Colligative properties are properties that depend only upon the number.
SOLUTION CHEM 22 Colligative Property Worksheet Studypool
Give the molecular formula, the van’t hoff factor for the following ionic. The others you’ll most likely have to look up. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. Colligative properties are properties that depend only upon the number of solute atoms, ions, or molecules in a solution and not on the nature.
electrolytes and colligative properties worksheet
The others you’ll most likely have to look up. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. Find the solutions to three problems involving colligative properties of solutions, such as freezing point depression, boiling point. Colligative properties are properties that depend only upon the number of solute atoms, ions, or molecules in a.
Colligative Properties Worksheet Answer Key Worksheet
The others you’ll most likely have to look up. Give the molecular formula, the van’t hoff factor for the following ionic. Practice colligative properties and phase diagrams with this worksheet that includes problems and solutions. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. Colligative properties are properties that depend only upon the number.
colligative properties worksheet chemfiesta com answers
We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. Practice colligative properties and phase diagrams with this worksheet that includes problems and solutions. Find the solutions to three problems involving colligative properties of solutions, such as freezing point depression, boiling point. Colligative properties are properties that depend only upon the number of solute atoms,.
Chemistry Colligative Properties Worksheet Practice Problems Answers
We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. Give the molecular formula, the van’t hoff factor for the following ionic. Colligative properties are properties that depend only upon the number of solute atoms, ions, or molecules in a solution and not on the nature of. Practice colligative properties and phase diagrams with this.
The Others You’ll Most Likely Have To Look Up.
Give the molecular formula, the van’t hoff factor for the following ionic. We expect you to know the solubility of lithium chlorate, lithium nitrate, and barium nitrate. Find the solutions to three problems involving colligative properties of solutions, such as freezing point depression, boiling point. Practice colligative properties and phase diagrams with this worksheet that includes problems and solutions.