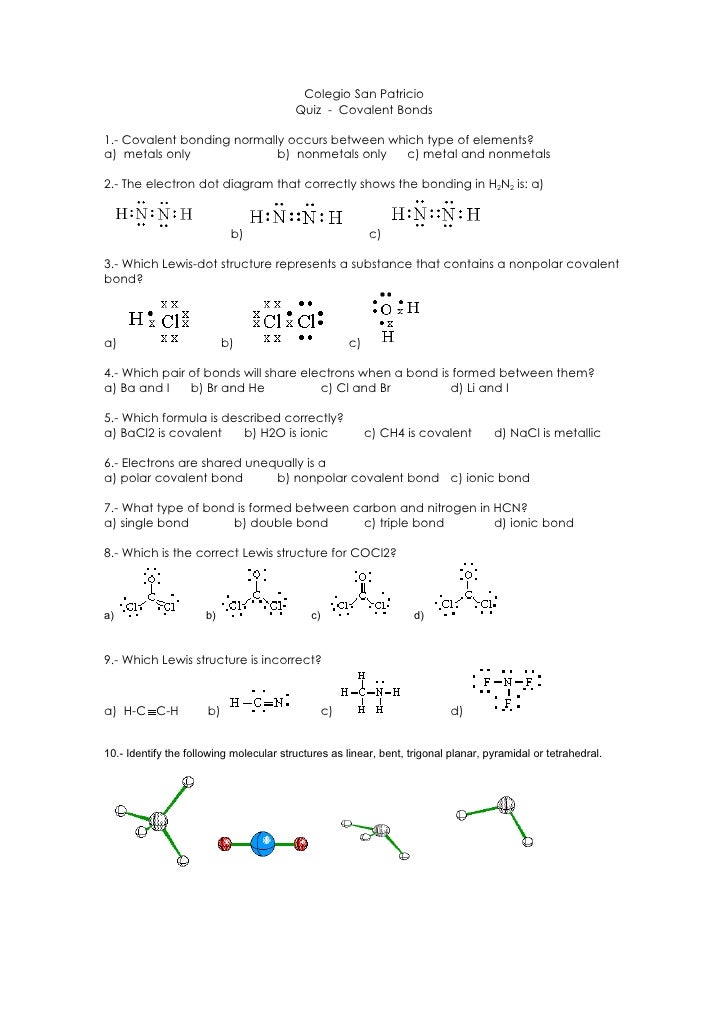
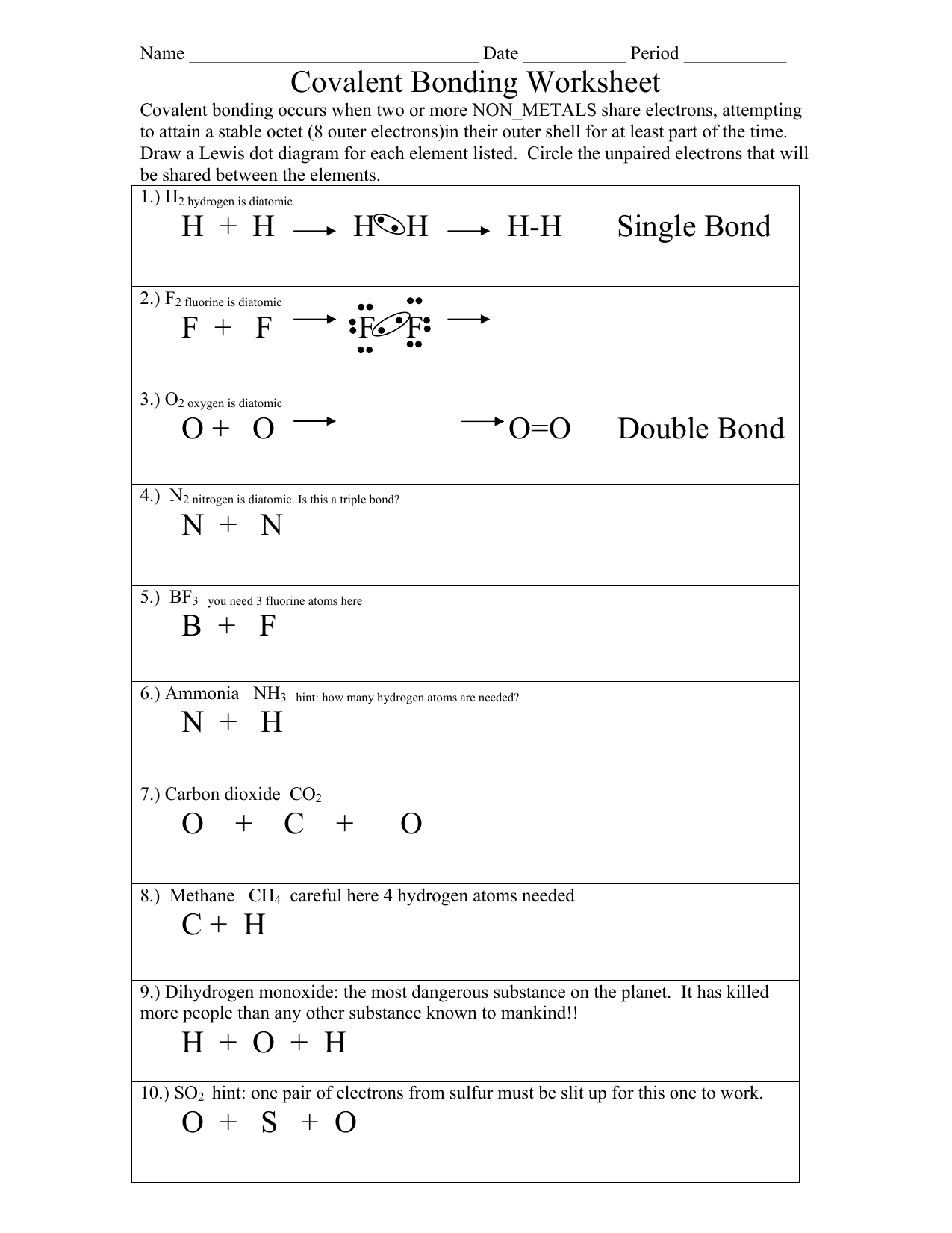
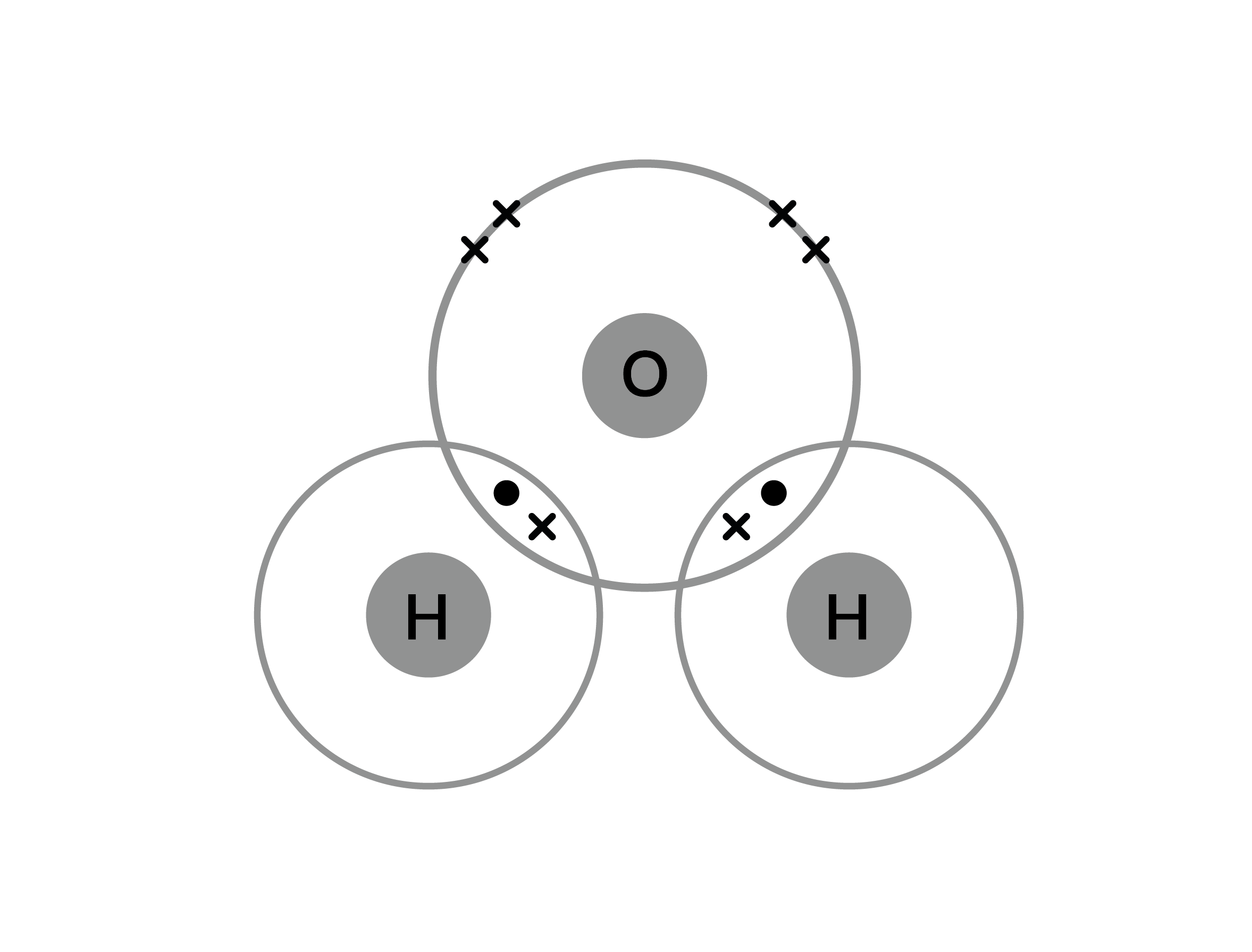
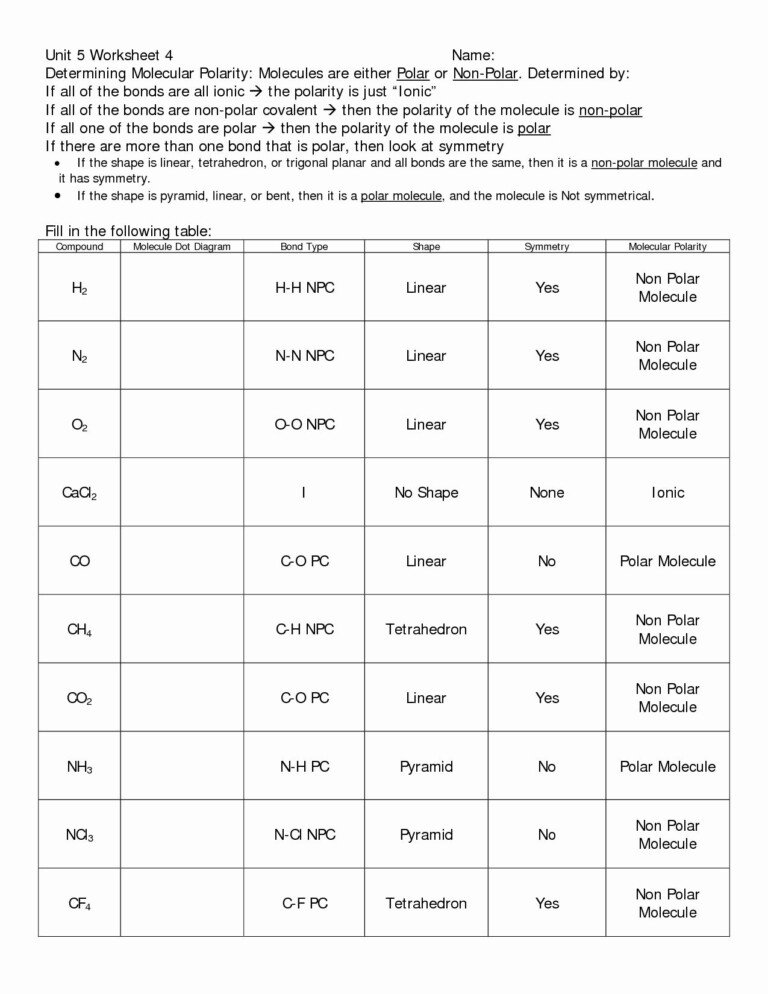
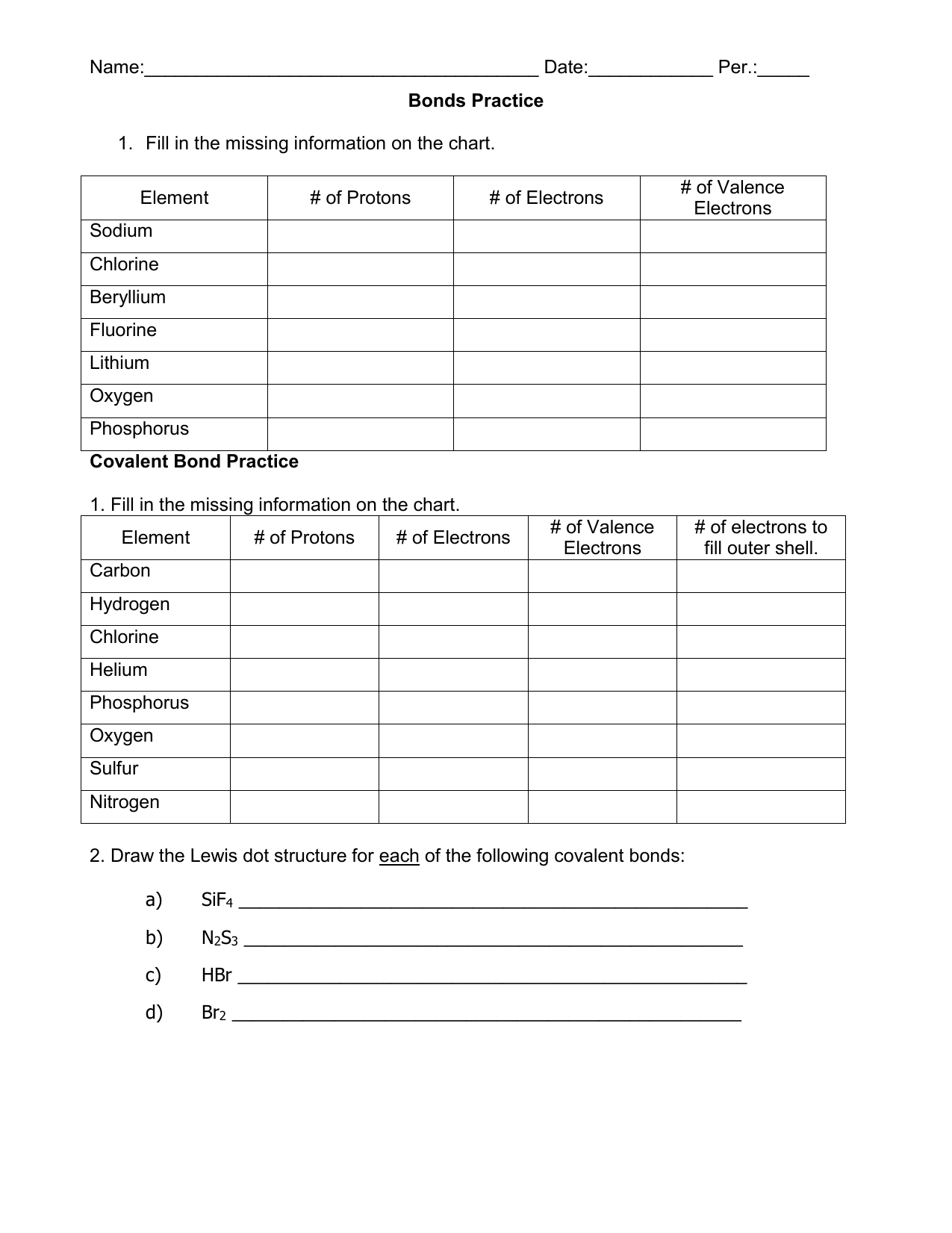
Covalent Bonding Practice Worksheet
Covalent Bonding Practice Worksheet - Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their.
Quiz Covalent Bonds
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow.
Covalent Bonding Worksheet
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow.
Covalent bonding Chemistry Explanation & Exercises evulpo
Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Quiz & Worksheet Covalent Chemical Bonds
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow.
Free Printable Covalent Bonding Worksheets Worksheets Library
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their.
Ionic And Covalent Bonding Practice Worksheet Worksheet
Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Naming Ionic And Covalent Bonds Practice
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Practice Drawing Covalent Bonds
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow.
Ionic And Covalent Bonding Practice Worksheet
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Covalent Bonding Naming Worksheet
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their.
Write A Simple Rule That Will Allow.
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.