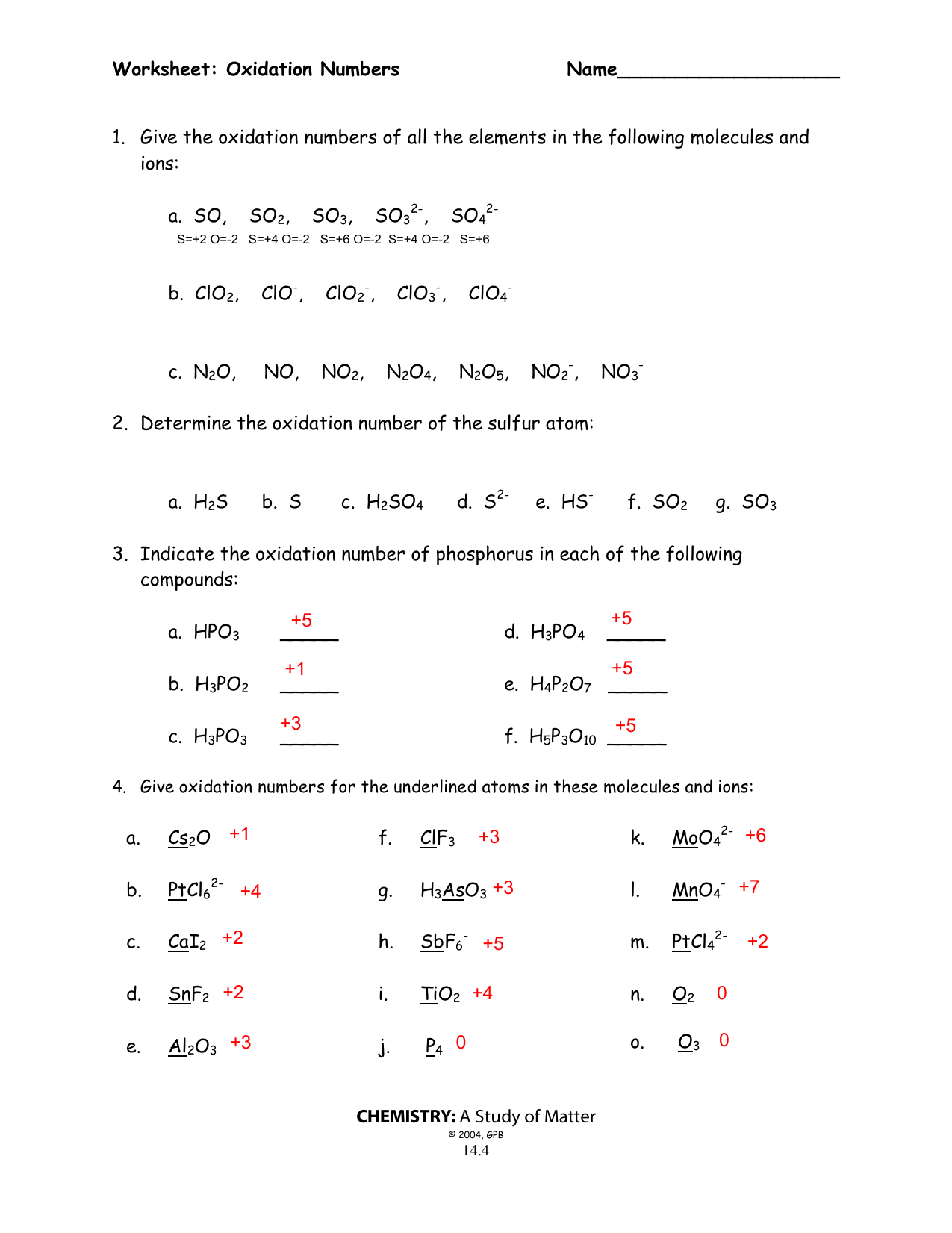
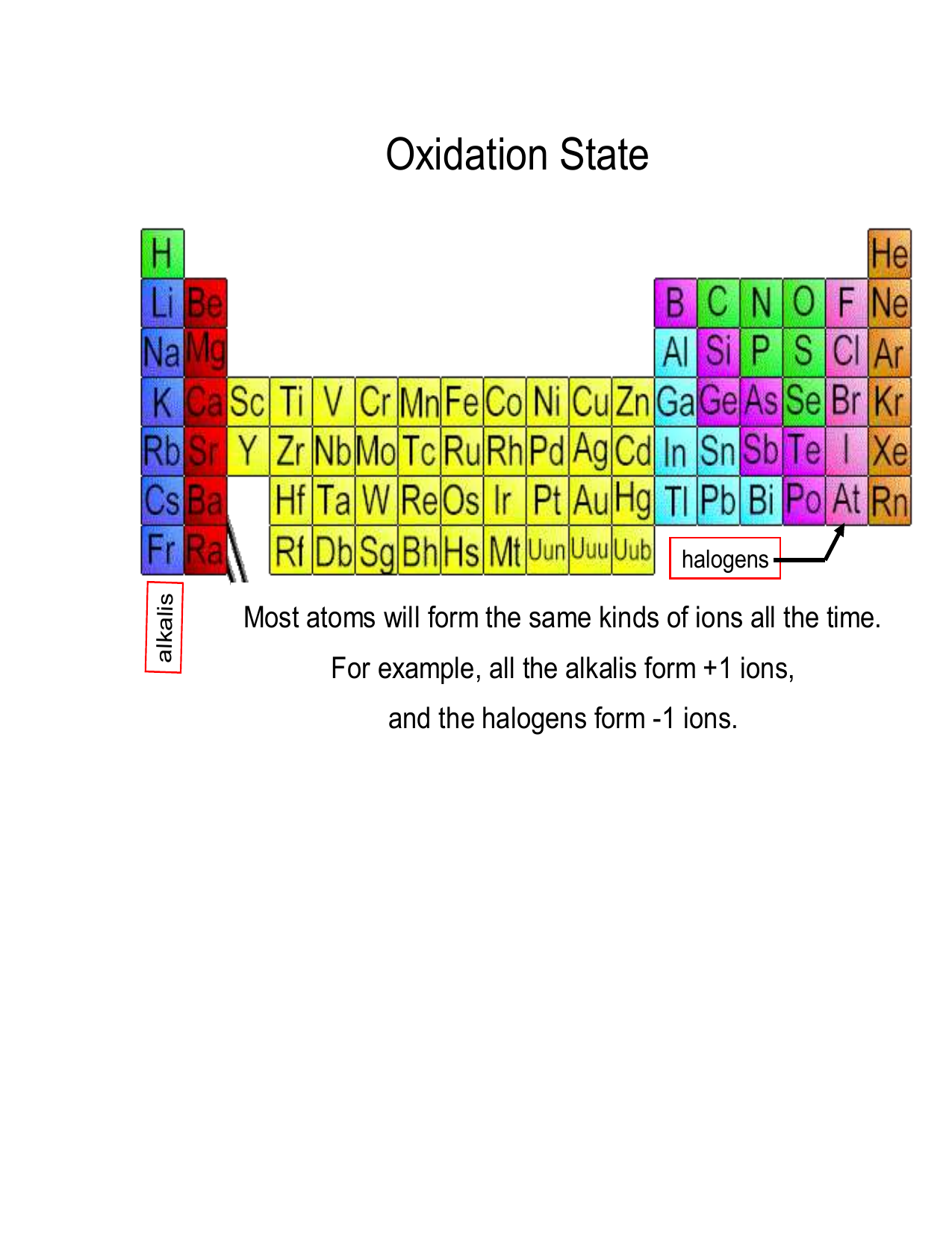
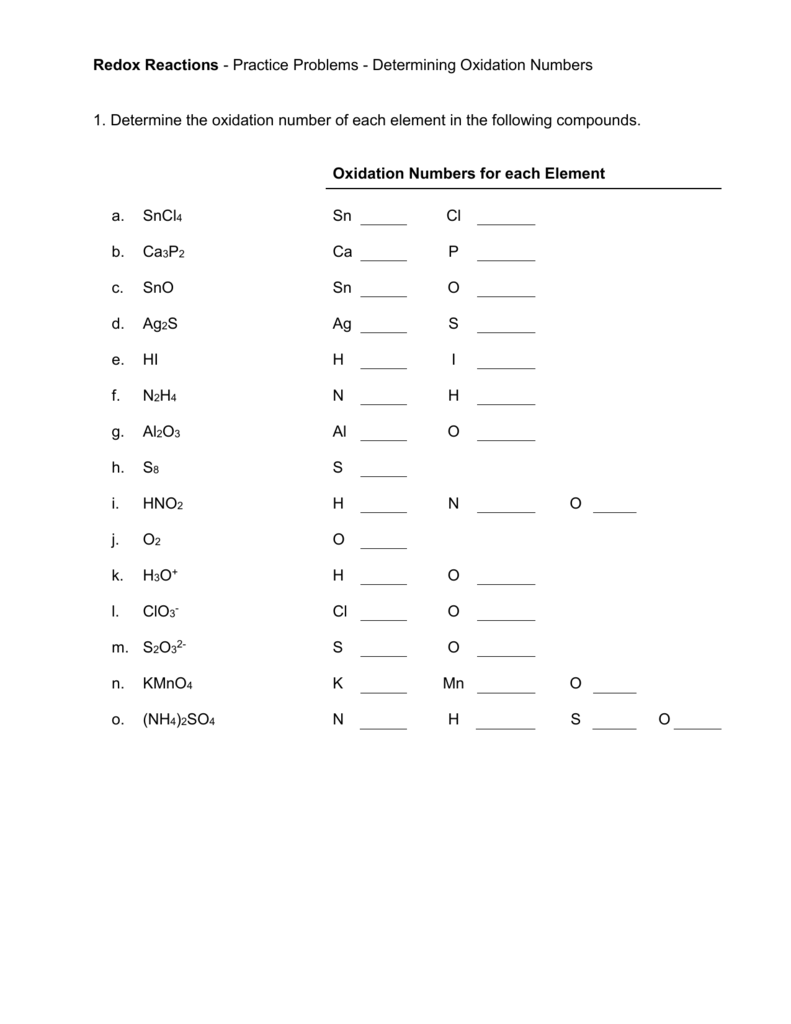
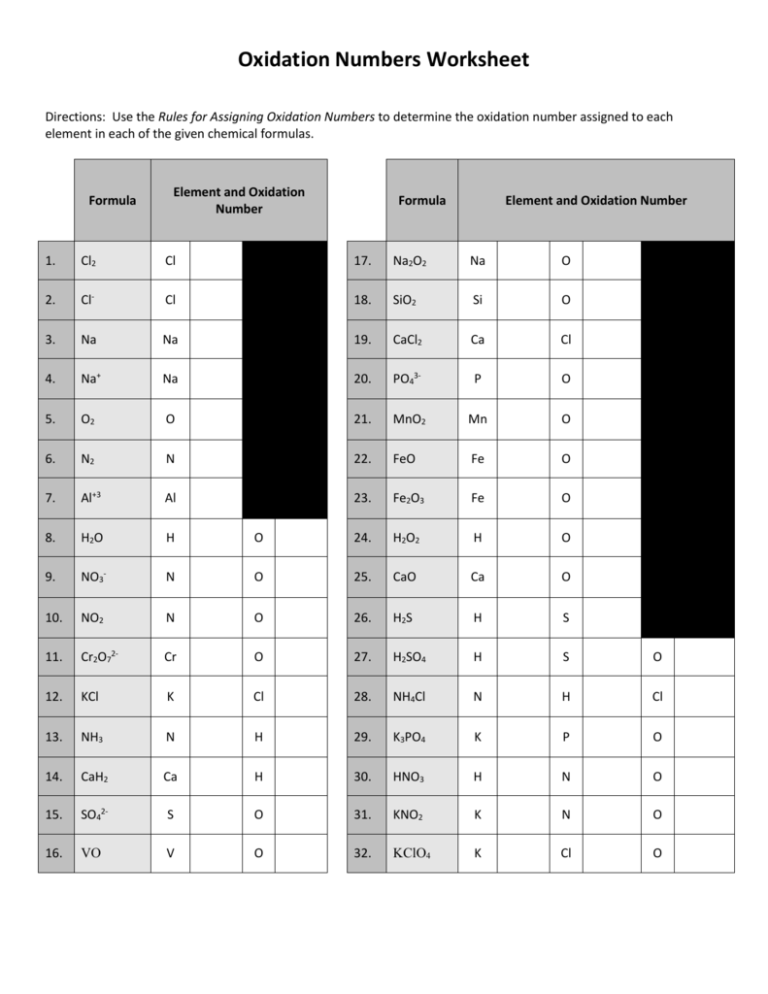
Worksheet Oxidation Numbers
Worksheet Oxidation Numbers - Oxidation numbers are bookkeeping numbers. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. In the following questions, give the oxidation number of the indicated atoms/ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. They mark the flow of electrons and are useful for balancing redox (reduction/oxidation). The oxidation number of an element in a monatomic ion equals the. 2) the oxidation number of a monatomic ion. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. A pure element has an oxidation number of 0.
Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. A pure element has an oxidation number of 0. Oxidation numbers are bookkeeping numbers. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. 2) the oxidation number of a monatomic ion. They mark the flow of electrons and are useful for balancing redox (reduction/oxidation). In the following questions, give the oxidation number of the indicated atoms/ion. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. The oxidation number of an element in a monatomic ion equals the. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules.
Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. The oxidation number of an element in a monatomic ion equals the. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. They mark the flow of electrons and are useful for balancing redox (reduction/oxidation). Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. A pure element has an oxidation number of 0. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. Oxidation numbers are bookkeeping numbers. 2) the oxidation number of a monatomic ion. In the following questions, give the oxidation number of the indicated atoms/ion.
Free Printable Oxidation Numbers Worksheets
Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. 2) the oxidation number of a monatomic ion. The oxidation number of an element in a monatomic ion equals the. Oxidation numbers are bookkeeping numbers.
Free Collection of Assigning Oxidation Numbers Worksheets
In the following questions, give the oxidation number of the indicated atoms/ion. They mark the flow of electrons and are useful for balancing redox (reduction/oxidation). Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. A pure element has an oxidation number of 0. 2) the oxidation number.
With this ColourbyNumber worksheet, chemistry or science students can
Oxidation numbers are bookkeeping numbers. A pure element has an oxidation number of 0. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. 2) the oxidation number of a monatomic ion. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above.
Free Printable Oxidation Numbers Worksheets Worksheets Library
Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. A pure element has an oxidation number of 0. They mark the flow of electrons and are useful for balancing redox (reduction/oxidation). 2).
Oxidation Numbers Worksheet
A pure element has an oxidation number of 0. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. In the following questions, give the.
Worksheet Oxidation Numbers Answer Key
Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. In the following questions, give the oxidation number of the indicated atoms/ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. Oxidation numbers are bookkeeping numbers. To determine the oxidation number of an element in a compound, use.
Assigning Oxidation Numbers Worksheet 1 Free Worksheets Printable
Oxidation numbers are bookkeeping numbers. The oxidation number of an element in a monatomic ion equals the. A pure element has an oxidation number of 0. 2) the oxidation number of a monatomic ion. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or.
07 Finding Oxidation Numbers Worksheet
A pure element has an oxidation number of 0. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. To determine the oxidation number of an element in a compound, use all the.
Oxidation Numbers Worksheet fill in the blank
Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. Oxidation numbers are bookkeeping numbers. A pure element has an oxidation number of 0. Working out oxidation numbers from electronegativity values, challenging.
Free Printable Oxidation Numbers Worksheets Worksheets Library
A pure element has an oxidation number of 0. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. 2) the oxidation number of a monatomic ion. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. Oxidation numbers are bookkeeping.
In The Following Questions, Give The Oxidation Number Of The Indicated Atoms/Ion.
2) the oxidation number of a monatomic ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. Oxidation numbers are bookkeeping numbers. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of.
They Mark The Flow Of Electrons And Are Useful For Balancing Redox (Reduction/Oxidation).
Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. The oxidation number of an element in a monatomic ion equals the. A pure element has an oxidation number of 0.